Imagine an individual who runs fairly slowly, has some trouble hearing, gets really sick when infection strikes, has aching joints, maybe some hair loss or wrinkly skin, and is not able to lift quite as much weight as she could a while back – this individual is clearly getting old, but is she an 80-year-old political leader, or a 45 year .old gorilla, or a 22 year old thoroughbred horse, or a 14 year old Labrador, or a 3 year old mouse?


All kinds of mammals – and birds, too – age in recognizably similar ways, but do so at very different rates, and the reasons for these differences are not yet understood. Young adult mice have the potential to live about 3 years, and dogs about 20 at most, but people usually do not suffer most aspects of aging for many decades, and whales for as much as 100 years or more.
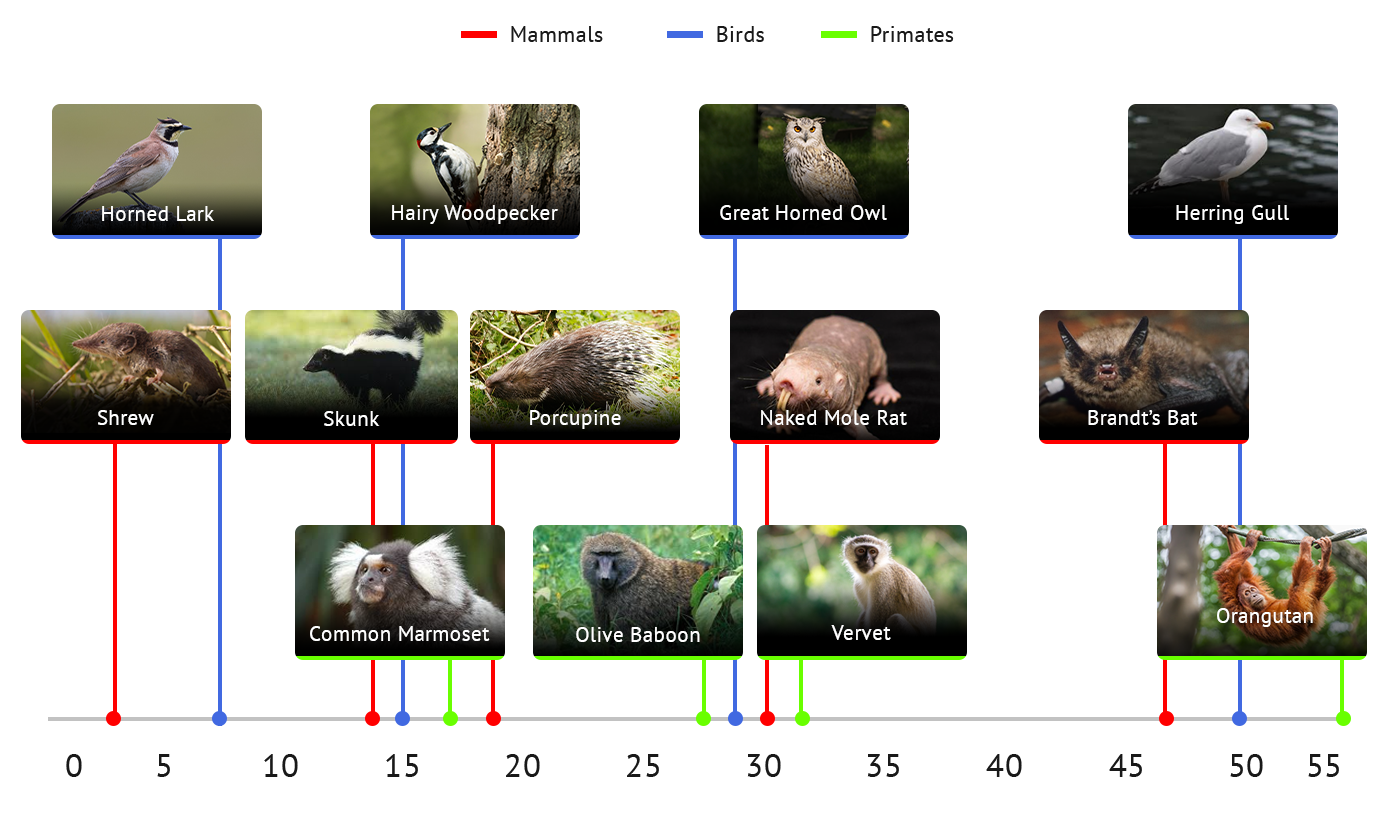
The fundamental premise of the cross-species (“comparative biogerontology”) component of the ILO project is that discovering the cellular and molecular basis for differences, among animal species, in the rate at which they age, i.e. the interval over which they can stay relatively young and healthy, will provide powerful new insights into pathways through which human aging, too, might be postponed. Such discoveries could point us to approaches for preserving good health and youthful function to ages at which most people have begun to show major age-related problems and multiple forms of illness.


The key element of our plan is to collect tissues from many species of rodents, primates, birds, and other varieties of mammals, and evaluate these samples using thepowerful tools of modern systems biology, focusing initially on small molecules (“metabolites”) and on cellular proteins. By using four independent sets of animals we will be able to find patterns of proteins and metabolites that discriminate long-lived species from others to which they are rel ated by ancestry. These will then serve as key targets for identification of drugs that can promote the same pathways in people.